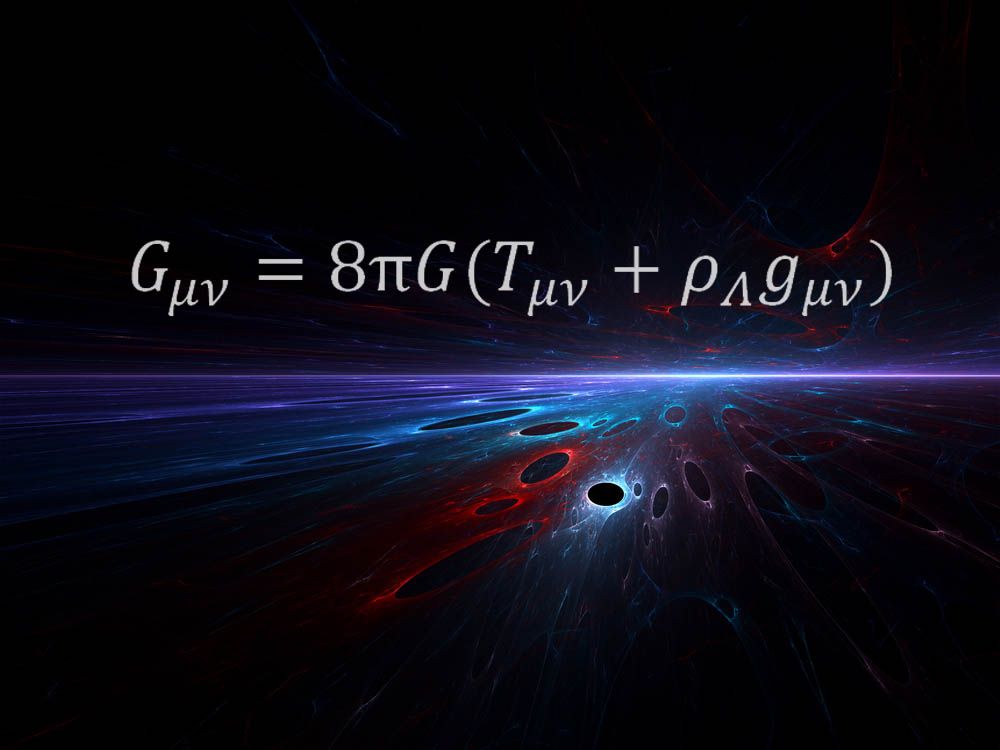S Universe Equation . \[\delta s_{universe}=\delta s+\delta \hat{s}\ge 0 \nonumber \] the equality applies when the process is reversible; equation \ref{2nd} is very important because it allows us to describe the second law of thermodynamics in terms of the system (chemical reaction), and. the second law of thermodynamics states that a spontaneous process increases the entropy of the universe, s univ > 0. ∆suniverse = ∆ssystem + ∆ssurroundings entropy and heat simplest case is a. “the entropy of the universe is always increasing.” in equation form: general relativity can tell us physically what factors relate to the expansion. One version of the einstein equations is the.
from www.livescience.com
general relativity can tell us physically what factors relate to the expansion. ∆suniverse = ∆ssystem + ∆ssurroundings entropy and heat simplest case is a. the second law of thermodynamics states that a spontaneous process increases the entropy of the universe, s univ > 0. \[\delta s_{universe}=\delta s+\delta \hat{s}\ge 0 \nonumber \] the equality applies when the process is reversible; equation \ref{2nd} is very important because it allows us to describe the second law of thermodynamics in terms of the system (chemical reaction), and. “the entropy of the universe is always increasing.” in equation form: One version of the einstein equations is the.
The 11 most beautiful mathematical equations Live Science
S Universe Equation “the entropy of the universe is always increasing.” in equation form: the second law of thermodynamics states that a spontaneous process increases the entropy of the universe, s univ > 0. general relativity can tell us physically what factors relate to the expansion. ∆suniverse = ∆ssystem + ∆ssurroundings entropy and heat simplest case is a. “the entropy of the universe is always increasing.” in equation form: One version of the einstein equations is the. \[\delta s_{universe}=\delta s+\delta \hat{s}\ge 0 \nonumber \] the equality applies when the process is reversible; equation \ref{2nd} is very important because it allows us to describe the second law of thermodynamics in terms of the system (chemical reaction), and.
From www.reddit.com
Equations written by Albert Einstein in 1931, describing the expansion S Universe Equation ∆suniverse = ∆ssystem + ∆ssurroundings entropy and heat simplest case is a. \[\delta s_{universe}=\delta s+\delta \hat{s}\ge 0 \nonumber \] the equality applies when the process is reversible; equation \ref{2nd} is very important because it allows us to describe the second law of thermodynamics in terms of the system (chemical reaction), and. “the entropy of the universe is. S Universe Equation.
From wallpapersafari.com
Maxwell's Equations Wallpaper WallpaperSafari S Universe Equation \[\delta s_{universe}=\delta s+\delta \hat{s}\ge 0 \nonumber \] the equality applies when the process is reversible; the second law of thermodynamics states that a spontaneous process increases the entropy of the universe, s univ > 0. “the entropy of the universe is always increasing.” in equation form: equation \ref{2nd} is very important because it allows us to. S Universe Equation.
From www.researchgate.net
(PDF) The Equation of the Universe S Universe Equation general relativity can tell us physically what factors relate to the expansion. “the entropy of the universe is always increasing.” in equation form: the second law of thermodynamics states that a spontaneous process increases the entropy of the universe, s univ > 0. \[\delta s_{universe}=\delta s+\delta \hat{s}\ge 0 \nonumber \] the equality applies when the process. S Universe Equation.
From www.pinterest.com
Science equation poster How the universe works, Physics S Universe Equation One version of the einstein equations is the. general relativity can tell us physically what factors relate to the expansion. the second law of thermodynamics states that a spontaneous process increases the entropy of the universe, s univ > 0. \[\delta s_{universe}=\delta s+\delta \hat{s}\ge 0 \nonumber \] the equality applies when the process is reversible; equation. S Universe Equation.
From www.livescience.com
The Universe Is Mathematics, Physicist Says Live Science S Universe Equation equation \ref{2nd} is very important because it allows us to describe the second law of thermodynamics in terms of the system (chemical reaction), and. ∆suniverse = ∆ssystem + ∆ssurroundings entropy and heat simplest case is a. \[\delta s_{universe}=\delta s+\delta \hat{s}\ge 0 \nonumber \] the equality applies when the process is reversible; general relativity can tell us physically. S Universe Equation.
From www.youtube.com
Thermodynamics 9.4 The Entropy Change of the Universe YouTube S Universe Equation “the entropy of the universe is always increasing.” in equation form: general relativity can tell us physically what factors relate to the expansion. equation \ref{2nd} is very important because it allows us to describe the second law of thermodynamics in terms of the system (chemical reaction), and. the second law of thermodynamics states that a spontaneous. S Universe Equation.
From physics.stackexchange.com
quantum field theory Equation of everything Physics Stack Exchange S Universe Equation “the entropy of the universe is always increasing.” in equation form: ∆suniverse = ∆ssystem + ∆ssurroundings entropy and heat simplest case is a. equation \ref{2nd} is very important because it allows us to describe the second law of thermodynamics in terms of the system (chemical reaction), and. general relativity can tell us physically what factors relate to. S Universe Equation.
From www.pinterest.com
The Universe Equation Answer to life, Universe, Cosmic microwave S Universe Equation equation \ref{2nd} is very important because it allows us to describe the second law of thermodynamics in terms of the system (chemical reaction), and. the second law of thermodynamics states that a spontaneous process increases the entropy of the universe, s univ > 0. One version of the einstein equations is the. \[\delta s_{universe}=\delta s+\delta \hat{s}\ge 0. S Universe Equation.
From exoplanets.nasa.gov
Are we alone in the universe? Revisiting the Drake equation S Universe Equation equation \ref{2nd} is very important because it allows us to describe the second law of thermodynamics in terms of the system (chemical reaction), and. \[\delta s_{universe}=\delta s+\delta \hat{s}\ge 0 \nonumber \] the equality applies when the process is reversible; the second law of thermodynamics states that a spontaneous process increases the entropy of the universe, s univ. S Universe Equation.
From www.pinterest.com
Schrodinger Wave Equation. The beauty of it. From the book "Quantum A S Universe Equation equation \ref{2nd} is very important because it allows us to describe the second law of thermodynamics in terms of the system (chemical reaction), and. \[\delta s_{universe}=\delta s+\delta \hat{s}\ge 0 \nonumber \] the equality applies when the process is reversible; “the entropy of the universe is always increasing.” in equation form: One version of the einstein equations is. S Universe Equation.
From www.pinterest.com.mx
The 11 Most Beautiful Mathematical Equations Mathematical equations S Universe Equation general relativity can tell us physically what factors relate to the expansion. the second law of thermodynamics states that a spontaneous process increases the entropy of the universe, s univ > 0. One version of the einstein equations is the. ∆suniverse = ∆ssystem + ∆ssurroundings entropy and heat simplest case is a. \[\delta s_{universe}=\delta s+\delta \hat{s}\ge 0. S Universe Equation.
From medium.com
The Most Important Equation In The Universe by Ethan Siegel Starts S Universe Equation general relativity can tell us physically what factors relate to the expansion. ∆suniverse = ∆ssystem + ∆ssurroundings entropy and heat simplest case is a. One version of the einstein equations is the. equation \ref{2nd} is very important because it allows us to describe the second law of thermodynamics in terms of the system (chemical reaction), and. “the. S Universe Equation.
From www.livescience.com
The 11 most beautiful mathematical equations Live Science S Universe Equation ∆suniverse = ∆ssystem + ∆ssurroundings entropy and heat simplest case is a. general relativity can tell us physically what factors relate to the expansion. “the entropy of the universe is always increasing.” in equation form: \[\delta s_{universe}=\delta s+\delta \hat{s}\ge 0 \nonumber \] the equality applies when the process is reversible; One version of the einstein equations is. S Universe Equation.
From www.yumpu.com
Friedmann Equation Newtonian derivation Astronomy S Universe Equation “the entropy of the universe is always increasing.” in equation form: One version of the einstein equations is the. general relativity can tell us physically what factors relate to the expansion. \[\delta s_{universe}=\delta s+\delta \hat{s}\ge 0 \nonumber \] the equality applies when the process is reversible; equation \ref{2nd} is very important because it allows us to. S Universe Equation.
From profoundphysics.com
The Friedmann Equations Explained A Complete Guide Profound Physics S Universe Equation “the entropy of the universe is always increasing.” in equation form: equation \ref{2nd} is very important because it allows us to describe the second law of thermodynamics in terms of the system (chemical reaction), and. \[\delta s_{universe}=\delta s+\delta \hat{s}\ge 0 \nonumber \] the equality applies when the process is reversible; the second law of thermodynamics states. S Universe Equation.
From www.businessinsider.com
Standard model of the cosmos as a math equation Business Insider S Universe Equation ∆suniverse = ∆ssystem + ∆ssurroundings entropy and heat simplest case is a. general relativity can tell us physically what factors relate to the expansion. equation \ref{2nd} is very important because it allows us to describe the second law of thermodynamics in terms of the system (chemical reaction), and. “the entropy of the universe is always increasing.” in. S Universe Equation.
From www.pinterest.com
The Universe Equation (With images) Universe, Equation, Cosmic S Universe Equation One version of the einstein equations is the. \[\delta s_{universe}=\delta s+\delta \hat{s}\ge 0 \nonumber \] the equality applies when the process is reversible; equation \ref{2nd} is very important because it allows us to describe the second law of thermodynamics in terms of the system (chemical reaction), and. general relativity can tell us physically what factors relate to. S Universe Equation.
From www.pinterest.com
The 11 most beautiful mathematical equations Mathematical equations S Universe Equation general relativity can tell us physically what factors relate to the expansion. ∆suniverse = ∆ssystem + ∆ssurroundings entropy and heat simplest case is a. the second law of thermodynamics states that a spontaneous process increases the entropy of the universe, s univ > 0. “the entropy of the universe is always increasing.” in equation form: One version. S Universe Equation.